Original Articles
Vol. 1 No. 2 (2022)
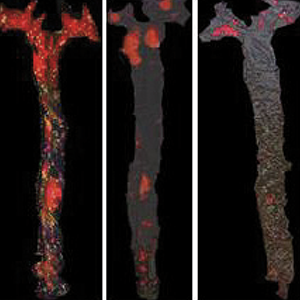
A nitric oxide-donor pravastatin hybrid drug exerts antiplatelet and antiatherogenic activity in mice
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Received: 9 February 2022
Accepted: 6 May 2022
Accepted: 6 May 2022
4871
Views
347
Downloads
Similar Articles
- PO51 | Thrombocythemia associated with a non-canonical JAK2 mutation: a case report , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- Roger Lijnen, Désiré Collen, The key to fibrinolysis and thrombolysis , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. 3 (2025)
- Eleonora Petito, Anna Maria Mezzasoma, Emanuela Falcinelli, Chiara Conti, Erica De Candia, Raimondo De Cristofaro, Silvia Sorrentino, Gian Marco Podda, Mariangela Scavone, Anna Falanga, Marina Marchetti, Luca Barcella, Stefania Basili, Lucia Stefanini, Andrea Boccatonda, Laura Contino, Patrizia Sciancalepore, Igor Florio, Egidio Imbalzano, Rossella Marcucci, Angela Rogolino, Patrizia Noris, Marta Panella, Rita Santoro, Maria Costanza Turi, Gaetano Vaudo, Rosa Curcio, Paolo Gresele, Persistence of functional anti-PF4 antibodies and neutrophil activation in vaccine-induced immune thrombotic thrombocytopenia , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. 3 (2025)
- Giovanni de Gaetano, When a feminist platelet went to the convention of prostaglandins and thromboxanes , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. 3 (2025)
- PO26 | Non-canonical coagulation platelets function: monocyte-platelet interaction a bridge between inflammation and coagulation , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO79 | Trying to hit the hit in the shadow of renal failure: a complex case managed with danaparoid , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- PO91 | Disseminated intravascular coagulation with deep vein thrombosis in a frail patient: an extreme manifestation triggered by sepsis during SGLT2i therapy , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- CO43 | Early platelet dysfunction in sepsis: an ICU pilot study , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
- Giovanni de Gaetano, Artificial intelligence, platelets and aspirin , Bleeding, Thrombosis and Vascular Biology: Vol. 3 No. 1 (2024)
- PO58 | Gender differences in primary haemostasis , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. s1 (2025)
1-10 of 193
Next
You may also start an advanced similarity search for this article.
Most read articles by the same author(s)
- Daniela Poli, Emilia Antonucci, Gualtiero Palareti, Roberto Facchinetti, Pietro Falco, Giuseppina Serricchio, Teresa Lerede, Lucilla Masciocco, Paolo Gresele, Sophie Testa, Major bleedings in mechanical prosthetic heart valves patients on Vitamin K antagonist treatment. Data from the PLECTRUM Study , Bleeding, Thrombosis and Vascular Biology: Vol. 1 No. 2 (2022)
- Paolo Gresele, Artificial intelligence and machine learning in hemostasis and thrombosis , Bleeding, Thrombosis and Vascular Biology: Vol. 2 No. 4 (2023)
- Loredana Bury, Paolo Gresele, The amazing genetic complexity of anucleated platelets , Bleeding, Thrombosis and Vascular Biology: Vol. 1 No. 2 (2022)
- Roberto Mario Santi, Annamaria Fulghesu, Ezio Zanon, Erica De Candia, Elvira Grandone, Giancarlo Di Renzo, Claudia Succu, Valentina Tosto, Vincenzina Bruni, Paolo Gresele, Diagnosis and management of abnormal uterine bleeding in adolescence , Bleeding, Thrombosis and Vascular Biology: Vol. 3 No. 1 (2024)
- Paolo Gresele, Synthetic platelets: can bioengineering realize in few years what evolution made in over 200 million years? , Bleeding, Thrombosis and Vascular Biology: Vol. 3 No. 3 (2024)
- Loredana Bury, Marco Malvestiti, Paolo Gresele, The 2024 Nobel prize in Medicine: impact on hemostasis and thrombosis research , Bleeding, Thrombosis and Vascular Biology: Vol. 3 No. 3 (2024)
- Stefania Momi, Paolo Gresele, Blood platelets and Charles Darwin’s natural selection , Bleeding, Thrombosis and Vascular Biology: Vol. 2 No. 1 (2023)
- Loredana Bury, Alessio Branchini, Francesco Bernardi, Paolo Gresele, Platelet transcriptomic changes in myocardial infarction are sex and clinical subtype-related: a step forward towards precision medicine? , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. 1 (2025)
- Eleonora Petito, Anna Maria Mezzasoma, Emanuela Falcinelli, Chiara Conti, Erica De Candia, Raimondo De Cristofaro, Silvia Sorrentino, Gian Marco Podda, Mariangela Scavone, Anna Falanga, Marina Marchetti, Luca Barcella, Stefania Basili, Lucia Stefanini, Andrea Boccatonda, Laura Contino, Patrizia Sciancalepore, Igor Florio, Egidio Imbalzano, Rossella Marcucci, Angela Rogolino, Patrizia Noris, Marta Panella, Rita Santoro, Maria Costanza Turi, Gaetano Vaudo, Rosa Curcio, Paolo Gresele, Persistence of functional anti-PF4 antibodies and neutrophil activation in vaccine-induced immune thrombotic thrombocytopenia , Bleeding, Thrombosis and Vascular Biology: Vol. 4 No. 3 (2025)