Original Articles
Vol. 1 No. 3 (2022)
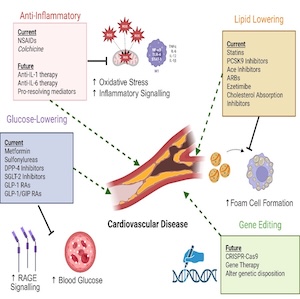
Crosstalk between hemostasis inhibitors and cholesterol biomarkers in multiple sclerosis
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Received: 31 May 2022
Accepted: 8 September 2022
Accepted: 8 September 2022
2300
Views
330
Downloads
Similar Articles
- Mattia Galli, C. Michael Gibson, Dominick J. Angiolillo, Factor XI inhibitors in adjunct to antiplatelet therapy: the ultimate dual-pathway inhibition? , Bleeding, Thrombosis and Vascular Biology: Vol. 2 No. 3 (2023)
- Daniela Poli, Walter Ageno, Emilia Antonucci, Salvatore Bradamante, Eugenio Bucherini, Paolo Chiarugi, Antonio Chistolini, Benilde Cosmi, Anna Falanga, Antonio Insana, Domenico Lione, Rosa Maria Lombardi, Giuseppe Malcangi, Rossella Marcucci, Giuliana Martini, Lucilla Masciocco, Carmelo Paparo, Daniele Pastori, Simona Pedrini, Vittorio Pengo, Pasquale Pignatelli, Andrea Toma, Sophie Testa, Gualtiero Palareti, Management of anticoagulation in atrial fibrillation patients in Italy: insight from the Atrial Fibrillation-Survey on Anticoagulated Patients Register (AF-START) , Bleeding, Thrombosis and Vascular Biology: Vol. 2 No. 2 (2023)
- Bianca Clerici, Mariangela Scavone, Gian Marco Podda, Recent advances in classic heparin-induced thrombocytopenia (HIT), autoimmune HIT, spontaneous HIT, and vaccine-induced immune thrombotic thrombocytopenia , Bleeding, Thrombosis and Vascular Biology: Vol. 3 No. 2 (2024)
- Betül Ünlü, Neha Joshi, Jamie M. O'Sullivan, Endothelial cell dysfunction in cancer: a not-so-innocent bystander , Bleeding, Thrombosis and Vascular Biology: Vol. 3 No. s1 (2024)
- Rita Carlotta Santoro, Roberto Minici, Marzia Leotta, Mariapia Falbo, Lucia Concetta Elia, Francesca Leo, Antonella Ierardi, Alessandra Strangio, Simona Prejanò, Vaccine-induced immune thrombotic thrombocytopenia following ChAdOx1 nCoV-19 vaccine: report of two cases , Bleeding, Thrombosis and Vascular Biology: Vol. 1 No. 2 (2022)
- Luca Barcella, Chiara Ambaglio, Paolo Gritti, Francesca Schieppati, Varusca Brusegan, Eleonora Sanga, Marina Marchetti, Luca Lorini, Anna Falanga, Long-term persistence of high anti-PF4 antibodies titer in a challenging case of AZD1222 vaccine-induced thrombotic thrombocytopenia , Bleeding, Thrombosis and Vascular Biology: Vol. 2 No. 2 (2023)
- Federica Azzolini, Antonio Bruno, Ettore Dolcetti, Diego Centonze, Fabio Buttari, Tumor necrosis factor superfamily in multiple sclerosis: from pathology to therapeutic implications , Bleeding, Thrombosis and Vascular Biology: Vol. 2 No. 2 (2023)
- Fabio Tumminello, Silvia Cardi, Corrado Lodigiani, Maria Elisa Mancuso, Antithrombotic therapy in idiopathic infertility , Bleeding, Thrombosis and Vascular Biology: Vol. 3 No. 3 (2024)
- Sergio Coccheri, A re-appraisal of thrombogenesis in COVID-19, seen as a multiple "Complex system" , Bleeding, Thrombosis and Vascular Biology: Vol. 1 No. 3 (2022)
- Cristina Legnani, Michela Cini, Sophie Testa, Alberto Tosetto, Claudia Dellanoce, Stefania Bellesso, Giuseppe Carli, Ilaria Nichele, Laura Lissandrini, Serena Zorzi, Emilia Antonucci, Gualtiero Palareti, Evaluation of Coaguchek® Pro II coagulation testing device performance to assess direct oral anticoagulant action. The DOAC-CHECK study , Bleeding, Thrombosis and Vascular Biology: Vol. 1 No. 3 (2022)
1-10 of 71
Next
You may also start an advanced similarity search for this article.