Mini-reviews
3 May 2023
Vol. 2 No. 2 (2023)
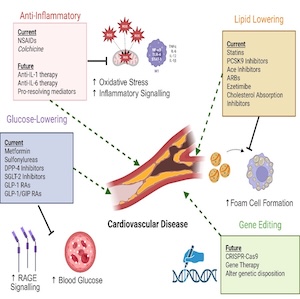
Tumor necrosis factor superfamily in multiple sclerosis: from pathology to therapeutic implications
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
1062
Views
234
Downloads
Most read articles by the same author(s)
- Ettore Dolcetti, Antonio Bruno, Diego Centonze, Is transient ischemic attack a minor stroke? , Bleeding, Thrombosis and Vascular Biology: Vol. 1 No. 1 (2022)