Long-term persistence of high anti-PF4 antibodies titer in a challenging case of AZD1222 vaccine-induced thrombotic thrombocytopenia
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Accepted: 1 June 2023
Authors
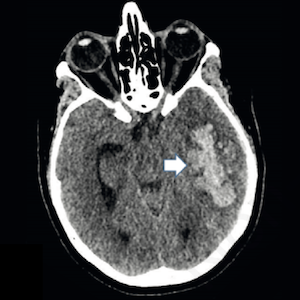
A syndrome occurring after adenoviral vector anti-SARS-CoV-2 vaccination, characterized by thrombocytopenia, venous thrombosis, and circulating anti-PF4 antibodies, known as vaccine-induced immune thrombotic thrombocytopenia (VITT), is well described. Data on the long-term course of this syndrome are lacking. Our aim is to report the clinical and laboratory features of a patient with VITT from diagnosis and during 21 months of follow-up. Cerebral venous thrombosis associated with elevated D-dimer, low fibrinogen, thrombocytopenia, and anti-PF4 antibodies positivity occurred in this patient after ChAdOx1 nCoV-19 vaccination. Cerebral thrombosis required a revascularization procedure and decompressive craniectomy. Upon dexamethasone and anticoagulant treatment initiation, the platelet count recovered. However, a persistently high anti-PF4 antibody titer, without thrombosis recurrence, was observed. Little is known about the long-term persistence of anti-PF4 antibodies, their clinical significance, and their possible role in guiding therapeutic decisions. In our patient, we decided to continue anticoagulant treatment beyond 21 months with parallel anti-PF4 antibody monitoring.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.